Markov State Modeling of Membrane Transport Proteins
Matthew C. Chan, Diwakar Shukla, Journal of Structural Biology, 213(4), 107800, (2021). DOI
Abstract
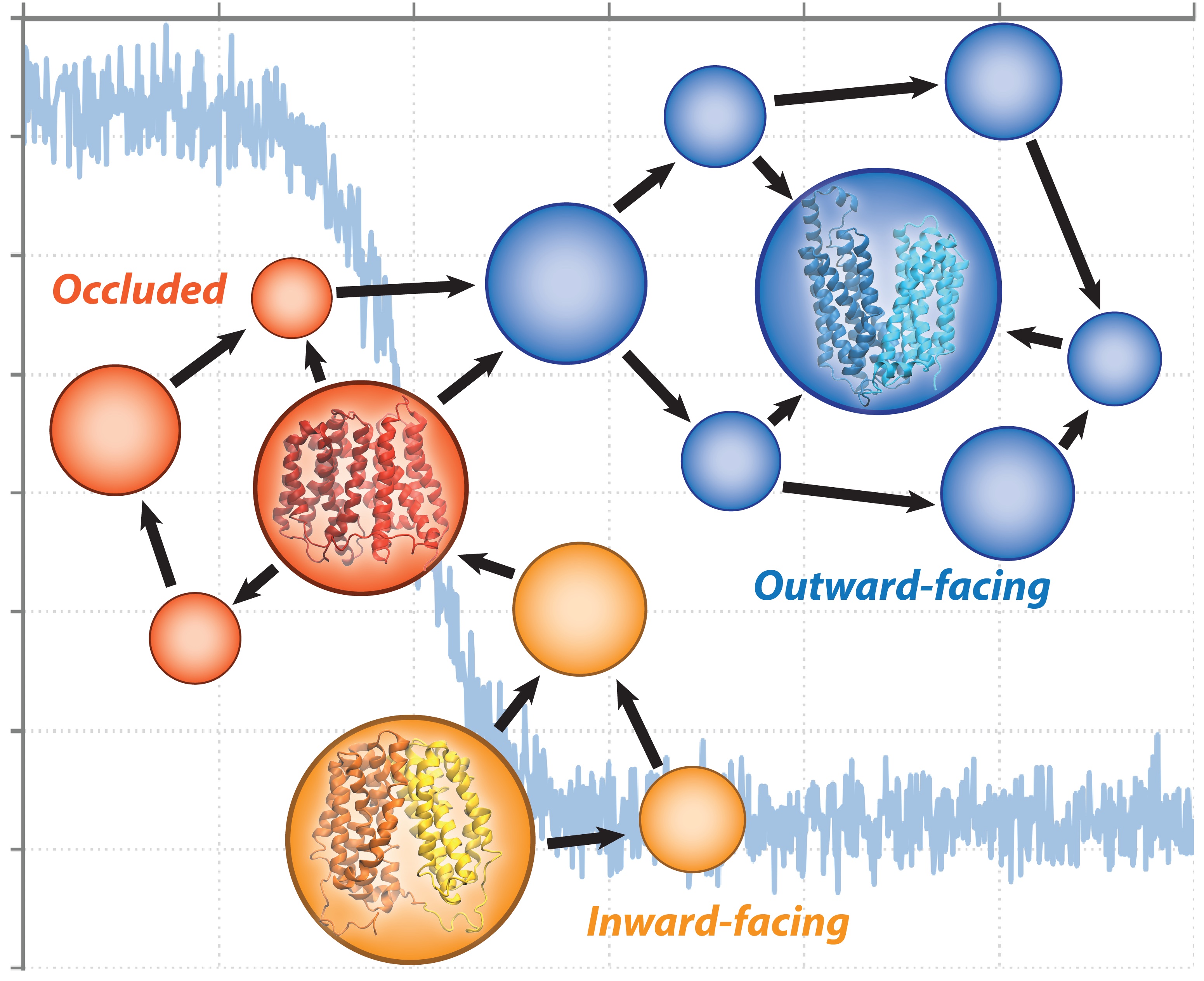
The serotonin transporter (SERT) initiates the reuptake of extracellular serotonin in the synapse to terminate neurotransmission. The cryogenic electron microscopy structures of SERT bound to ibogaine and the physiological substrate serotonin resolved in different states have provided a glimpse of the functional conformations at atomistic resolution. However, the conformational dynamics and structural transitions to intermediate states are not fully understood. Furthermore, the molecular basis of how serotonin is recognized and transported remains unclear. In this study, we performed unbiased microsecond-long simulations of the human SERT to investigate the structural dynamics to various intermediate states and elucidated the complete substrate import pathway. Using Markov state models, we characterized a sequential order of conformational-driven ion-coupled substrate binding and transport events and calculated the free energy barriers of conformation transitions associated with the import mechanism. We find that the transition from the occluded to inward-facing state is the rate-limiting step for substrate import and that the substrate decreases the free energy barriers to achieve the inward-facing state. Our study provides insights on the molecular basis of dynamics-driven ion-substrate recognition and transport of SERT that can serve as a model for other closely related neurotransmitter transporters.