The Effects of N-linked Glycosylation on SLC6 Transporters
M. C. Chan, D. Shukla, "The Effects of N-linked Glycosylation on SLC6 Transporters", Journal of Chemical Information and Modeling, 63(9), 2748-2758, (2023). DOI
Abstract
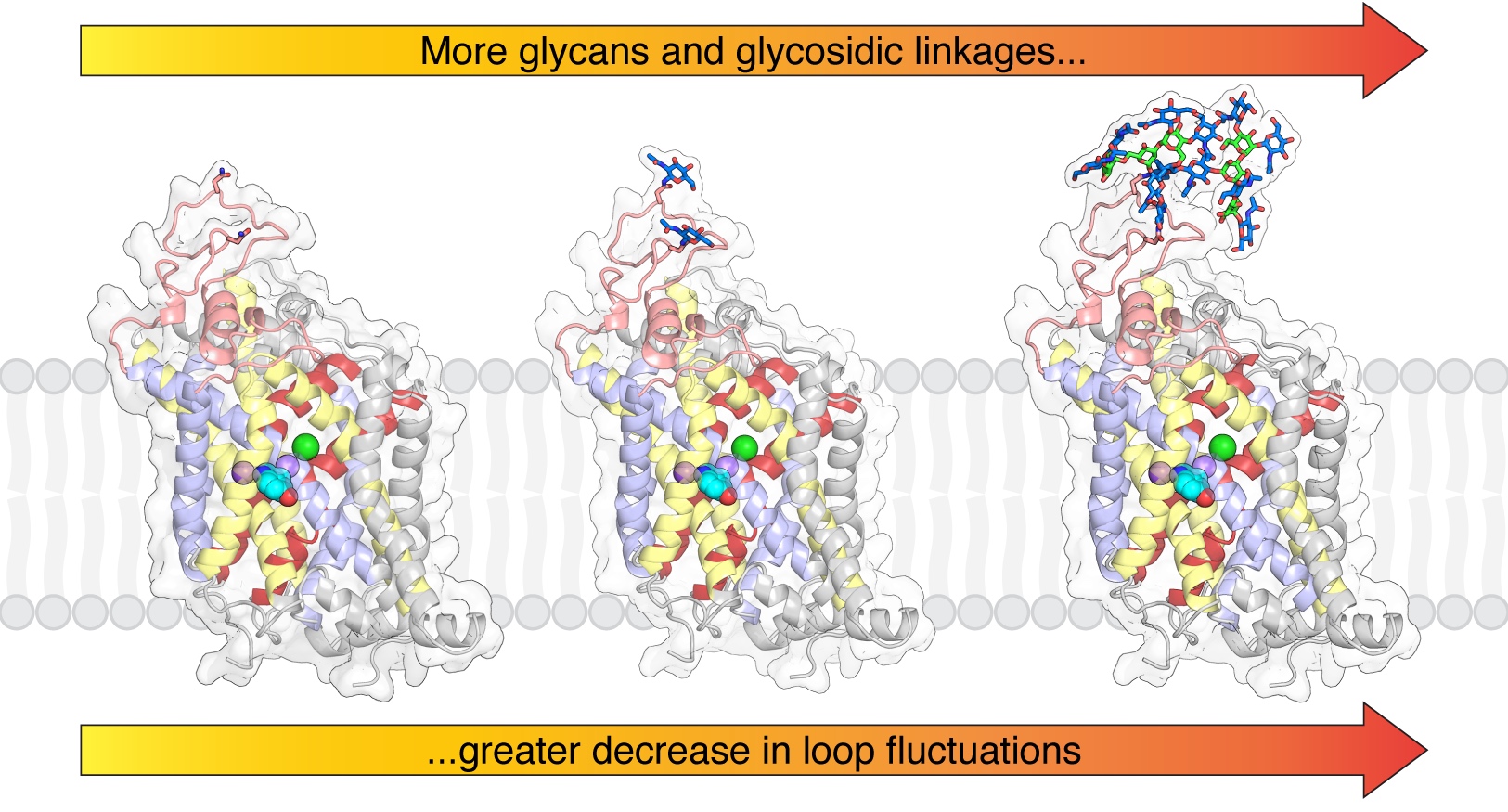
Membrane transporters of the solute carrier 6 (SLC6) family mediate various physiological processes by facilitating the translocation of amino acids, neurotransmitters, and other metabolites. In the body, the activity of these transporters is tightly controlled through various post-translational modifications with implications on protein expression, stability, membrane trafficking, and dynamics. While N-linked glycosylation is a universal regulatory mechanism among eukaryotes, a consistent mechanism of how glycosylation affects the SLC6 transporter family remains elusive. It is generally believed that glycans influence transporter stability and membrane trafficking; however, the role of glycosylation on transporter dynamics remains disputable, with differing conclusions among individual transporters across the SLC6 family. In this study, we collected over 1 ms of aggregated all-atom molecular dynamics (MD) simulation data to systematically identify the impact of N-glycans on SLC6 transporter dynamics. We modeled four human SLC6 transporters, the serotonin, dopamine, glycine, and B0AT1 transporters, by first simulating all possible combinations of a glycan attached to each glycosylation site followed by investigating the effect of larger, oligo-N-linked glycans to each transporter. The simulations reveal that glycosylation does not significantly affect the transporter structure but alters the dynamics of the glycosylated extracellular loop and surrounding regions. The structural consequences of glycosylation on the loop dynamics are further emphasized with larger glycan molecules attached. However, no apparent differences in ligand stability or movement of the gating helices were observed, and as such, the simulations suggest that glycosylation does not have a profound effect on conformational dynamics associated with substrate transport.