The Shape of Water in Zeolites and its Impact on Oxidation Catalysis
Daniel T. Bregante, Matthew C. Chan, Jun Zhi Tan, E. Zeynep Ayla, Christopher P. Nicholas, Diwakar Shukla, David W. Flaherty, Nature Catalysis, 4(9), 797-808, (2021). DOI
Abstract
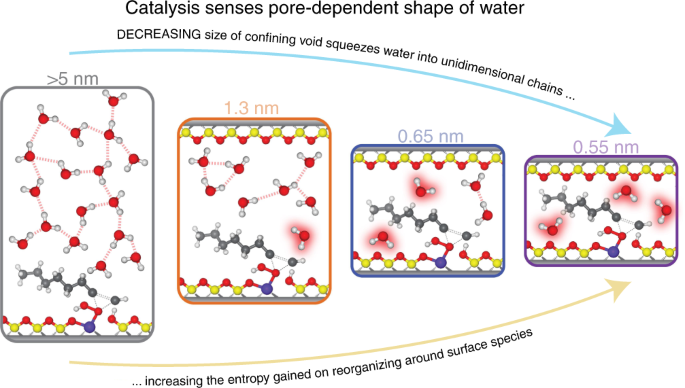
Solvent structures that surround active sites reorganize during catalysis and influence the stability of surface intermediates. Within the pores of a zeolite, H2O molecules form hydrogen-bonded structures that differ significantly from bulk H2O. Spectroscopic measurements and molecular dynamics simulations show that H2O molecules form bulk-like three-dimensional structures within 1.3 nm cages, while H2O molecules coalesce into oligomeric one-dimensional chains distributed throughout zeolite frameworks when the pore diameter is smaller than 0.65 nm. The differences between the motifs of these solvent structures provide opportunities to manipulate enthalpy-entropy compensation relationships and significantly increase rates of catalytic turnover events. Here, we explain how the reorganization of these pore size-dependent H2O structures during alkene epoxidation catalysis gives rise to entropy gains that increase turnover rates by up to 400-fold. Collectively, this work shows how solvent molecules form discrete structures with highly correlated motion within microporous environments, and that the reorganization of these structures may be controlled to confer stability to reactive intermediates.